Learn more about the science of Microscope Photometry
When photons interact with matter, such as when light is focused onto a sample in a microscope, it can either be reflected, absorbed and then re-emitted, or scattered. Photometry measures the intensity of light interactions, within a certain wavelength range, between photons and a sample.
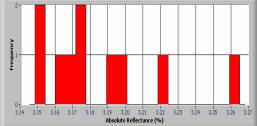
Histogram of photometric data
Absorption
Spectroscopy is the study of the quantized energy transfer between radiation and matter and photometry is the study of the intensity of that transfer. The energy of light is directly related to its wavelength by the Einstein-Planck relationship:
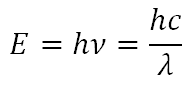
where E is the energy of the photon, h is Planck's constant: 6.626 x 10-34 J∙s, ν is the frequency of the photon, c is the speed of light: 3.0 x 108 m/s, and λ is the wavelength of the photon.
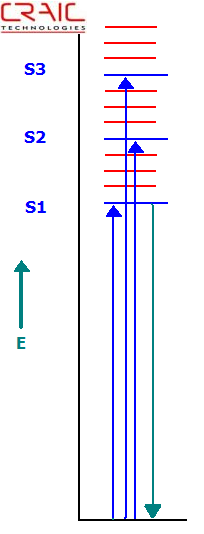
The electromagnetic energy of a photon is inversely proportional to its wavelength. In other words, short wavelength or blue light is higher in energy than longer wavelengths or red light. Due to these energetic differences, photons with different wavelengths interact differently with molecules. In the ultraviolet-visible region, electronic transitions are mainly observed. Namely, when a photon of the proper energy is absorbed by a molecule, an electron is excited to higher energy level or shell. This is most commonly described in what is called a Jablonski diagram, or an energy level diagram.
For a photon to be absorbed, the energy of the photon must be exactly the same as the difference in energy between the ground state and the excited state to which the electron transfers. As can be seen in the diagram, the electron jumps to the first excited state (S1) when an electron of the corresponding energy is absorbed. If a photon of a higher energy, one that matched the difference between the ground and say the second excited state (S2), were absorbed, the electron would jump from the ground state to the S2 state. This process is called absorption.
The energy levels of the molecules are due to the types of atoms and how they are bound to one another. Additionally, the shape of the molecule as well as its environment can also play a part in structuring the energy levels. In fact, a dye chemist can "tune" a dye molecule by adding or removing functional groups or atoms of a molecule, thereby changing its color.
Fluorescence
After the electron has jumped to the excited state, it usually then decays by internal conversion to the lowest excited state, S1. From there it can decay back to the ground state by a number of paths. The most common is a radiationless transition whereby the electron drops from the S1 excited state to the ground state losing energy without the emission of a photon. However, when the electron drops from the S1 state to the ground state with the emission of a photon, the process is called fluorescence. It is a rapid process and fluorescence lifetimes usually follow first order kinetic rules. It should be noted that the fluorescence intensity is governed by many factors, some of which include excitation wavelength, fluorescence quantum yield, quenching factors and even molecular structure.
Reflectance
Reflectance photometry simply measures the instensity of the light reflected from the sample. The portion that is not reflected may have been absorbed (see above), transmitted through the sample (if transparent to that wavelength of light) or even scattered. Reflected light may be divided into two types: specular and diffuse. Simply put, specular reflectance is like the reflectance from a mirror. The light is reflected at the same angle as it impinges upon the mirror surface. Diffuse reflectance is similar to what occurs with white paper where light is effectively reflected at all angles.
Jablonski Energy Level Diagram Depicting Absorbance and Fluorescence Transitions